
The complex in archaea is called the thermosome.Group II chaperonins (TCP-1), found in the eukaryotic cytosol and in archaea, are more poorly characterized. Group II Structure of Saccharomyces cerevisiae TRiC in the AMP-PNP bound state ( PDB: 5GW5). Some bacteria use multiple copies of this chaperonin, probably for different peptides. The crystal structure of Escherichia coli GroEL has been resolved to 2.8 Å. The RuBisCO subunit binding protein is a member of this family. The binding of cpn10 to cpn60 inhibits the weak ATPase activity of cpn60. The cpn10 and cpn60 oligomers also require Mg 2+-ATP in order to interact to form a functional complex. The Cpn60 subfamily was discovered in 1988. GroEL/GroES may not be able to undo protein aggregates, but kinetically it competes in the pathway of misfolding and aggregation, thereby preventing aggregate formation. It is like a cover that covers GroEL (box/bottle). GroES (is a single-ring heptamer that binds to GroEL in the presence of ATP or transition state analogues of ATP hydrolysis, such as ADP-AlF 3.
#CHAPERONE PROTEINS MITOCHONDRIA PATCH#
GroEL is a double-ring 14mer with a greasy hydrophobic patch at its opening and can accommodate the native folding of substrates 15-60 kDa in size.coli is a Group I chaperonin and the best characterized large (~ 1 MDa) chaperonin complex. Group I chaperonins (Cpn60) are found in bacteria as well as organelles of endosymbiotic origin: chloroplasts and mitochondria. Classification Group I GroES/GroEL complex (side) The original chaperonin is proposed to have evolved from a peroxiredoxin. Each ~60kDa peptide chain can be divided into three domains, apical, intermediate, and equatorial. Each ring is composed of either 7, 8 or 9 subunits depending on the organism in which the chaperonin is found. The structure of these chaperonins resemble two donuts stacked on top of one another to create a barrel. Chaperonin proteins may also tag misfolded proteins to be degraded.

Molecular chaperones catalyze protein refolding by accelerating partial unfolding of misfolded proteins, aided by energy supplied by the hydrolysis of adenosine triphosphate (ATP). Most proteins spontaneously fold into their most stable three-dimensional conformation, which is usually also their functional conformation, but occasionally proteins mis-fold. The energy to fold proteins is supplied by non-covalent interactions between the amino acid side chains of each protein, and by solvent effects. Newly made proteins usually must fold from a linear chain of amino acids into a three-dimensional tertiary structure. HSP60 belong to a large class of molecules that assist protein folding, called molecular chaperones.
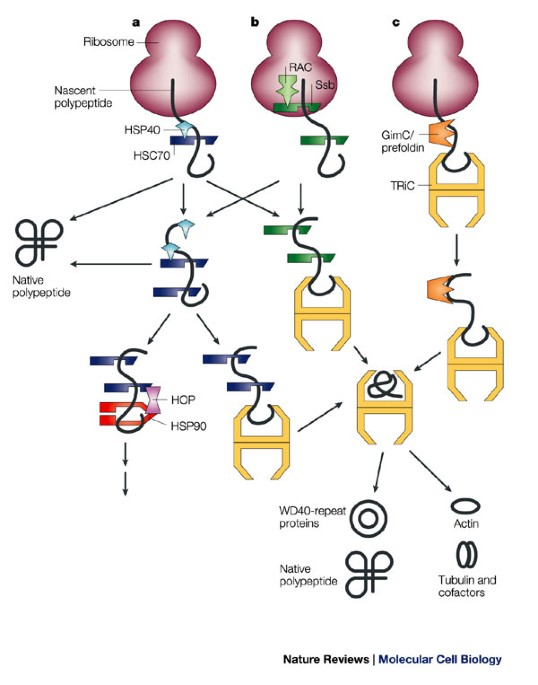
They prevent misfolding of proteins during stressful situations such as high heat, by assisting protein folding. HSP60, also known as chaperonins ( Cpn), is a family of heat shock proteins originally sorted by their 60kDa molecular mass. Structure of the bacterial chaperonin GroEL.


 0 kommentar(er)
0 kommentar(er)
